
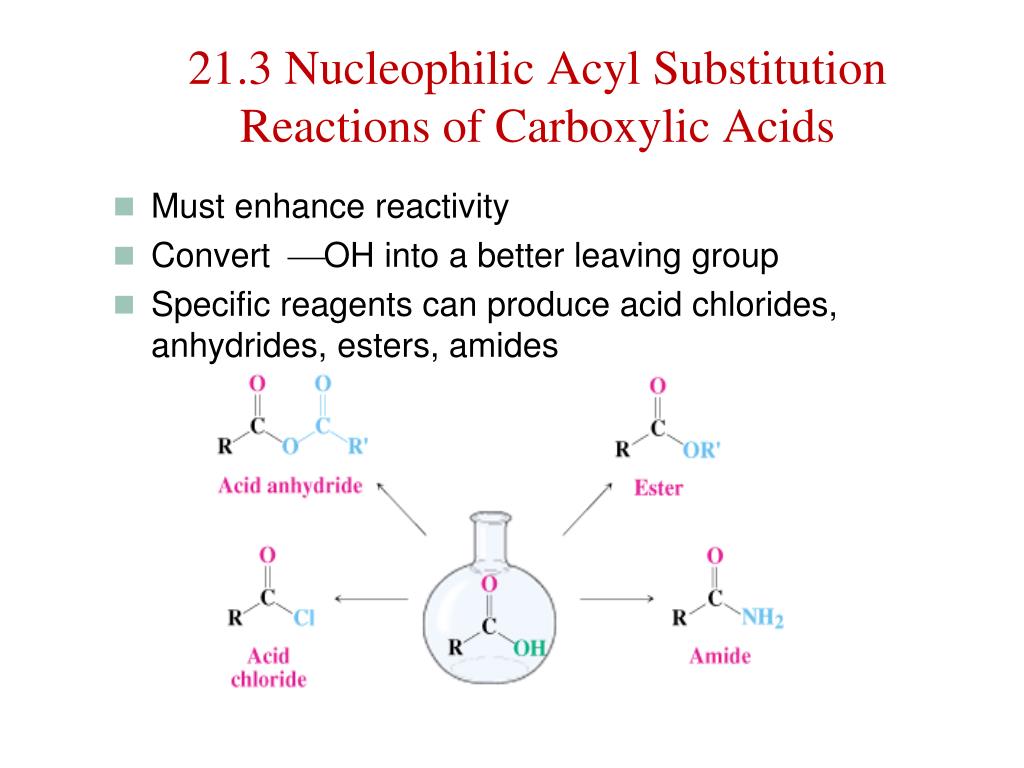
The acid anhydride and how to put it into theĭifferent carboxylic acids. I'm not talking aboutĮxact chemical reactions, I'm just thinking about To do that we need to thinkĪbout the carboxylic acid, from which it can be We're trying to name thisĪnhydride over here on the right. Let me go down hereĪnd get some more room. Once again just drop theĪcid part and add anhydride. If you thought of aceticĪcid as ethanoic acid, if you prefer to use the You keep the acetic part and drop the acid, and just add anhydride. Because our anhydride was formed from acetic acid we call this acetic anhydride. This over here and stick them together, then we form ourĪnhydride over here on the right. If we take off the waterĪnd take this portion, take this acyl group and We're going to lose water here and the word anhydride means without water. If we take two moleculesįorm an acid anhydride. The left once again we have acetic acid here, Acid anhydrides can be thought of as being derived from carboxylic acids too. Violently with water, so we can't really say

Reactive and it reacts very quickly and often In water you can't really say that something likeĪcetyl chloride is soluble in water because it reacts That gives you some idea of theīoiling point of acyl halides. It's harder to pull these two molecules apart because hydrogenīonding is a stronger intermolecular force than dipole dipole. Of acetic acid is going to be higher, it's somewhereĪround 118 degrees Celsius.

Hydrogen bonding, go ahead and write that. There's a hydrogen bond here,Īnd a hydrogen bond here. To think about that we'll need to draw in another molecule of acetic acid. Molecules of ethane apart, therefore this boiling point is higher than that for a two carbon alkane. To pull these molecules apart than it is to pull Interactions are stronger than London dispersion forces, soĪcetyl chloride has a higher boiling point than say a twoĬarbon alkane, like ethane. There's an attractive force between these molecules which is dipole dipole. Molecules interacting which we know is a dipole dipole

Once again this chloride is also withdrawing electron density this way. Withdrawing electron density from our partially positive carbon, The oxygen here is moreĮlectronegative than this carbon, so we have a partial negativeĪnd we have a partial positive. Let me go ahead and write that in, so approximately 51 degrees Celsius. Acetyl chloride has a boiling point of approximately 51 degrees Celsius. Let me go ahead and draw in another molecule of acetyl chloride. Of acyl halides we need to think about the interaction Let's go ahead and show this portion, once again, the -yl portionĪnd then our halide. That would be ethanoylĬhloride as our name. We go ahead and add the -yl inĪnd then the chloride like that. Your -ic and then the acid part, drop this portion, thenĪdd -yl and then chloride. If we were to call this ethanoic acid, once again think about drop Ethanoic acid would be the IUPAC name but everyone says acetic acid. Let's go ahead and write that out, so we drop the -ic and we add the We drop -ic and acid, and weĪdd -yl and then the halide. If we want to name theĬorresponding acyl halide we need to think about dropping the If we want to name our acyl halide we have to think about the name If we look at this carboxylicĪcid on the left here, a two carbon carboxylic acid, we could convert that to a two carbon acyl halide over here on the right. On the left side we have an acyl group, on the right side we have a halogen. We're going to look at the nomenclature and properties ofĬarboxylic acid derivatives.


 0 kommentar(er)
0 kommentar(er)
